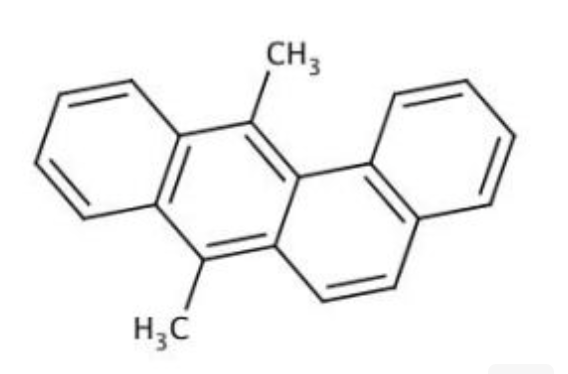
7,12-DIMETHYLBENZ[A]ANTHRACENE
CAS No. 57-97-6
7,12-DIMETHYLBENZ[A]ANTHRACENE( DMBZA | DMBA )
Catalog No. M23108 CAS No. 57-97-6
7,12-Dimethylbenz[a]anthracene is a highly potent carcinogen that is activated by microsomal enzymes to a diol epoxide metabolite that binds covalently to DNA in mammalian cells, leading ultimately to tumor induction.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | 55 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name7,12-DIMETHYLBENZ[A]ANTHRACENE
-
NoteResearch use only, not for human use.
-
Brief Description7,12-Dimethylbenz[a]anthracene is a highly potent carcinogen that is activated by microsomal enzymes to a diol epoxide metabolite that binds covalently to DNA in mammalian cells, leading ultimately to tumor induction.
-
Description7,12-Dimethylbenz[a]anthracene (DMBA) is a polycyclic aromatic hydrocarbon (PAH) that has been found in tobacco smoke and diesel exhaust and has carcinogenic activity.It undergoes metabolic activation by numerous enzymes, including the cytochrome P450 (CYP450) isoform CYP1B1, as well as microsomal epoxide hydrolase (mEH), producing a variety of reactive metabolites that form DNA adducts in vivo, and it has been commonly used to induce tumor formation in various rodent models.DMBA increases proliferation and migration of, and induces epithelial-to-mesenchymal transition (EMT) in, MCF-10A breast cancer cells when used at a concentration of 5 μM.It also has immunosuppressant activity, inhibiting proliferation of isolated mouse splenic B- and T cells stimulated ex vivo with LPS or concanavalin A, respectively, when administered at doses of 50 and 100 mg/kg.Intragastric administration of DMBA (20 mg/animal) induces formation of mammary gland tumors in rats.
-
In Vitro——
-
In Vivo——
-
SynonymsDMBZA | DMBA
-
PathwayOthers
-
TargetOther Targets
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number57-97-6
-
Formula Weight256.34
-
Molecular FormulaC20H16
-
Purity>98% (HPLC)
-
SolubilitySoluble in toluene (50 mg/ml), acetone, chloroform, dichloromethane, ethanol, diethyl ether, n-octanol, and hot water (very slightly). Insoluble in cold water.
-
SMILESCC1=C2C=CC3=CC=CC=C3C2=C(C4=CC=CC=C14)C
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Rengarajan, T., Rajendran, P., Nandakumar, N., et al. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 5(3), 182-189 (2015).
2. Csiszar, A., Balasubramanian, B., Tarantini, S., et al. Chemically induced carcinogenesis in rodent models of aging: Assessing organismal resilience to genotoxic stressors in geroscience research. Geroscience 41(2), 209-227 (2019).
3. Huberman, E., Chou, M.W., and Yang, S.K. Identification of 7,12-dimethylbenz[a]anthracene metabolites that lead to mutagenesis in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 76(2), 862-866 (1979).
molnova catalog



related products
-
Venuloside A
Venuloside A (ESK246), a monoprostane glycoside from Pittosporum venulosum, is a LAT3 inhibitor and a leucine uptake inhibitor that inhibits the transporter of leucine through LAT3, and may be used in the study of prostate cancer.
-
Sugammadex (sodium)
Sugammadex a synthetic derivative of γ-cyclodextrin is a steroid-based neuromuscular blocker reversing agent.
-
Enterodiol
Enterodiol has antioxidant and immunomodulatory effects, it is able to pass the intestinal barrier and modulate cytokine production.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


